Nearly all of the research themes prevalent in the Keillor group involve the study of the function, inhibition and engineering of enzymes. The projects described here are representative of our current research efforts in the research areas of medicinal chemistry, chemical biology and biocatalysis.
Medicinal Chemistry
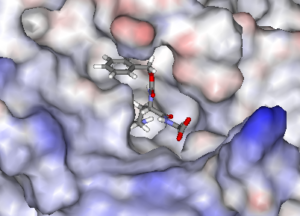
The unregulated activity of TGases has been implicated in a number of physiological disorders, including fibrosis, coeliac disease and cancer. We have discovered novel classes of potent irreversible inhibitors of tissue TGase that are under evaluation for their therapeutic potential in several types of cancer. These inhibitors are being refined and improved through further efforts in synthesis and kinetic evaluation.
Chemical Biology
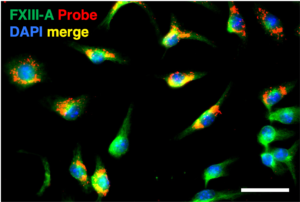
Transglutaminases (TGases) mediate the cross-linking of proteins by catalysing the formation of isopeptide bonds through a transamidation reaction between a Gln side chain and a Lys side chain. Although many details of the catalytic mechanism have been resolved, important questions still remain, particularly with respect to how the structure of the enzyme, known to undergo major and minor conformational changes, affect its function and biological role(s), in multiple sub-cellular contexts. We have designed chemical probes for interrogating the localisation and activity of TGases in cultured mammalian cells.
Biocatalysis
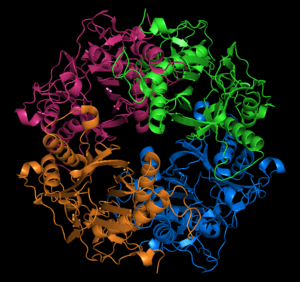
Nylon is an outstanding polymer that is used in many applications, but nylon waste has accumulated in the environment and is difficult to manage. Nylon hydrolases are enzymes that are capable of depolymerizing nylons, and show potential for application to biocatalytic remediation. We are developing novel small molecule assays as convenient surrogates for nylon hydrolysis activity, to be used in protein engineering efforts towards more efficient biocatalysts.
